Would The Resulting Solution Conduct Electricity
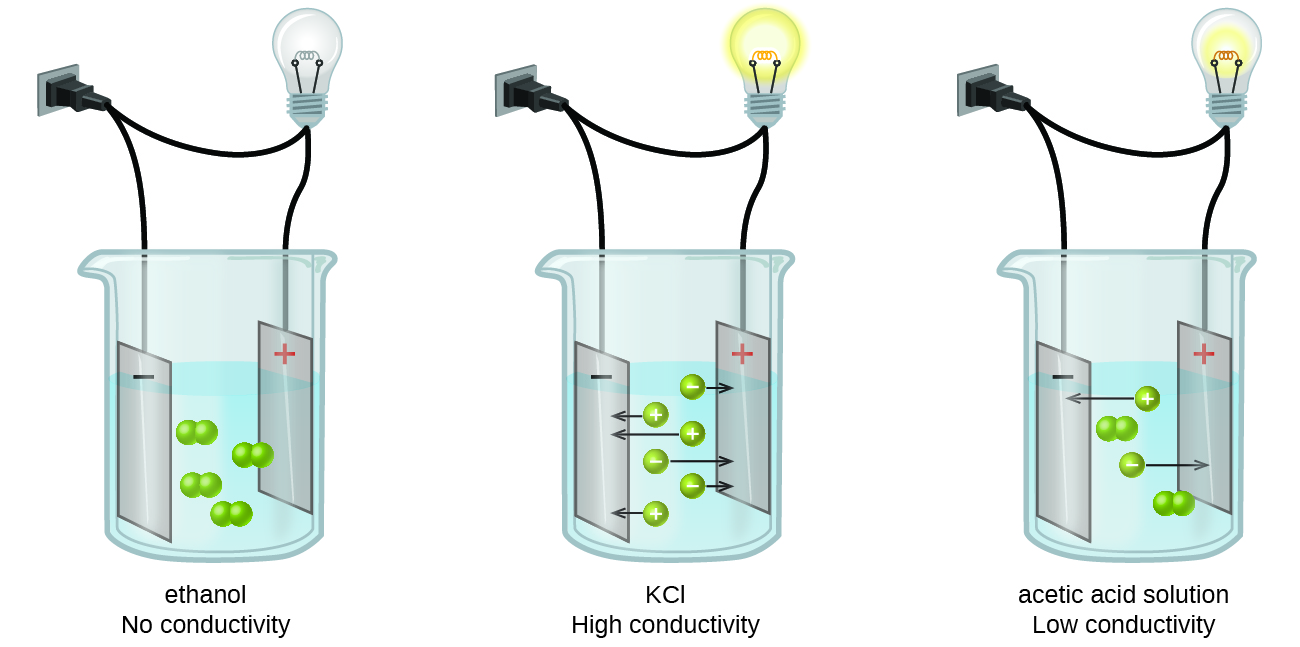
Solutions may also conduct electricity if they contain dissolved ions with conductivity increasing as ion concentration increases. Any substance that when dissolved in water allows the resulting solution to conduct electricity.

Safety Safety Ladder Get The Job
The resulting solution will conduct electricity because it contains ions.

Would the resulting solution conduct electricity. But when impurities such as salt dissolve in water the resulting solution conducts electricity very well. A solubility table shows that almost all compounds of Group 1 metals are soluble. The resulting solution can conduct electricity when voltage is applied to it making electrolytes especially useful in batteries.
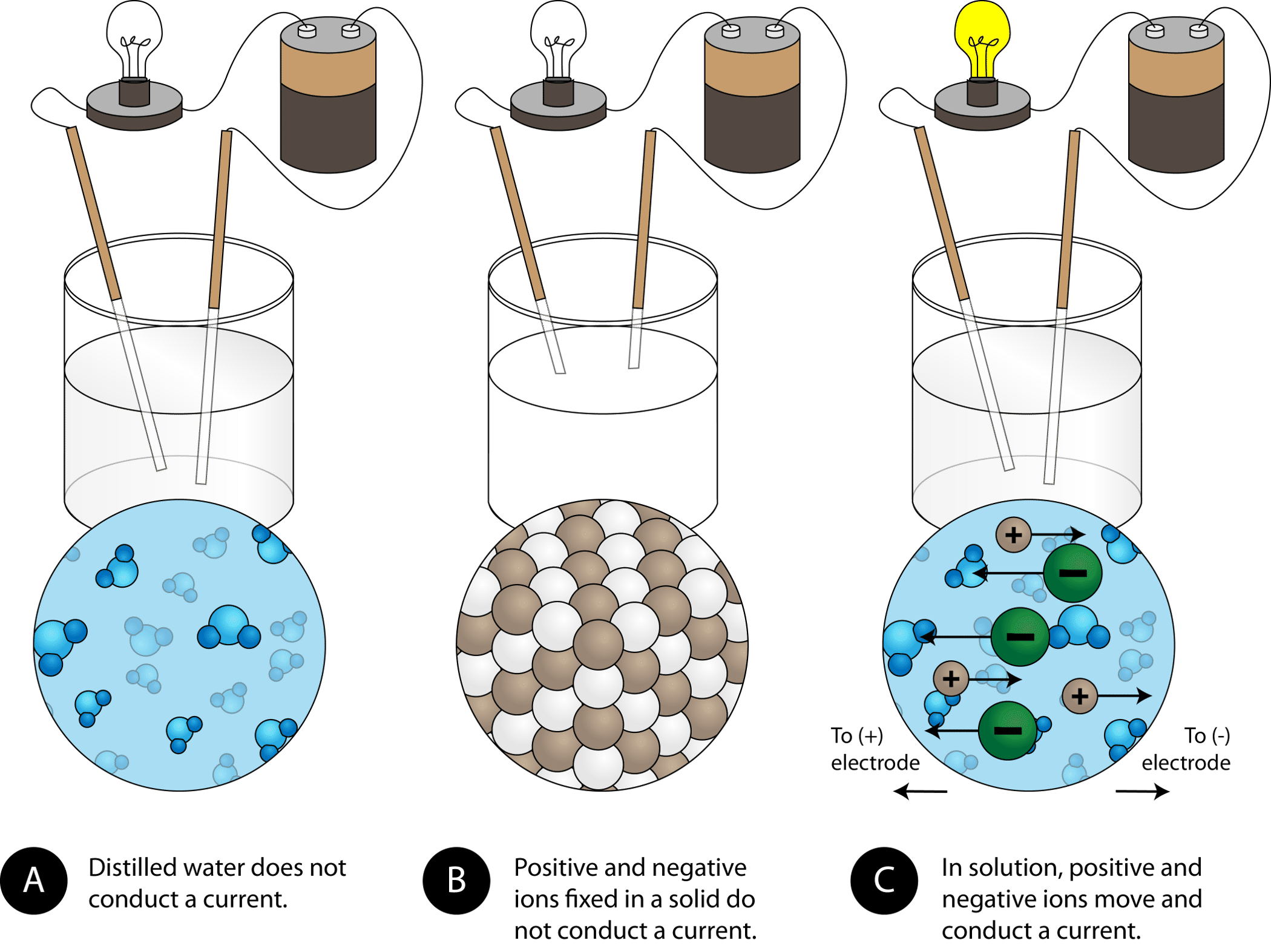
The resulting dissolved ions are electrically charged particles that allow the solution to conduct electricity. A pure substance does not conduct electricity in the solid state but it does dissolve in water and the resulting solution conducts electricity. Covalent substances can dissolve in water but cannot conduct electricity.
Most familiar is the conduction of electricity through metallic wires in which case the mobile charged entities are electrons. When salt is dissolved in water the resulting solution conducts electricity well. Out of these NaCl and CuSO4are salts H2SO4 and HCl is a strong acid and NaOH is a strong base.
The result is a solution with free moving charged particles able to conduct electricity Materials that dissolve in water to form a solution that will conduct electricity are called _________ electrolytes. To conduct electricity a substance must contain freely mobile charged species. Dissolving sodium chloride in water releases ions according to the equation.
Might dissolve in water but they cannot conduct electricity. Salts strong acids and strong bases dissociate almost completelyin their aqueous solutions. Any substance that when dissolved in water allows the resulting solution to conduct electricity.
This general rule tells you that. Do not release ions. Which statement explains why this occurs with these substances.
In fact its a very powerful conductor. Glucose solution do not conduct electricity. Cell is immersed in an electrolyte solution with a.
The resulting solution will conduct electricity C. The aqueous solutions of the compounds NaCl CuSO4 HCl H2SO4and NaOHare electricity conductors. The temperature of the water plays a large role in how well it conducts electricity.
Only compounds that dissociate into their component ions in solution qualify. The following chemical equations represent this phenomenon. Consists of two electrodes immersed in an electrolyte solution with a.
Pure water and pure salt are poor conductors of electricity. The process of dissolving makes the electrons in their atoms free to move. NaCl s Na 1 aq Cl 1 aq.
Hot water conducts better in general. The word electrolyte was coined in the 1800s from electro- electrical from the Greek root elektro and lytos or loosed in Greek. The fundamental apparatus used in electrochemistry.
Is hot water a good conductor of electricity. All of the above. Dissolving solid sodium chloride in water for example releases ions according to the dissociation equation.
The Effect of Concentration If an ionic compound is dissolved in water it dissociates into ions and the resulting solution will conduct electricity. The substance has a fairly high melting point. It is important to keep in mind however that CO 2 is not an electrolyte because CO 2 itself does not dissociate into ions.
NaCl s Na aq Cl- aq How do I write a similar balanced chemical equation for these electrolyte. NaCl s Na aq Cl-aq. If an ionic compound is dissolved in water it dissociates into ions and the resulting solution will conduct electricity.
All of the above. If an ionic compound is dissolved in water it dissociates into ions and the resulting solution will conduct electricity. Most of the acid remains as nonionized molecules in equilibrium with ions.

Which Metals Have High Electrical Conductivity Conductive Materials Electricity Electrical Efficiency

Mixtures Solids And Liquids Gumballs Chemistry Lessons Mixtures Gumball
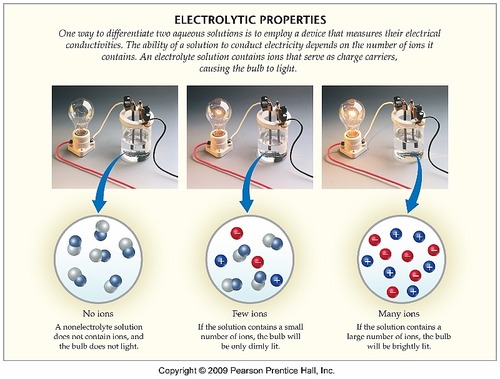
What Substances Conduct Electricity By The Movement Of Ions Socratic

Swot Analysis A Simple Way To Find Your Competitive Edge Plus A Free Template Swot Analysis Analysis Economy

Why Does Sodium Chloride Solution Conduct Electricity Quora
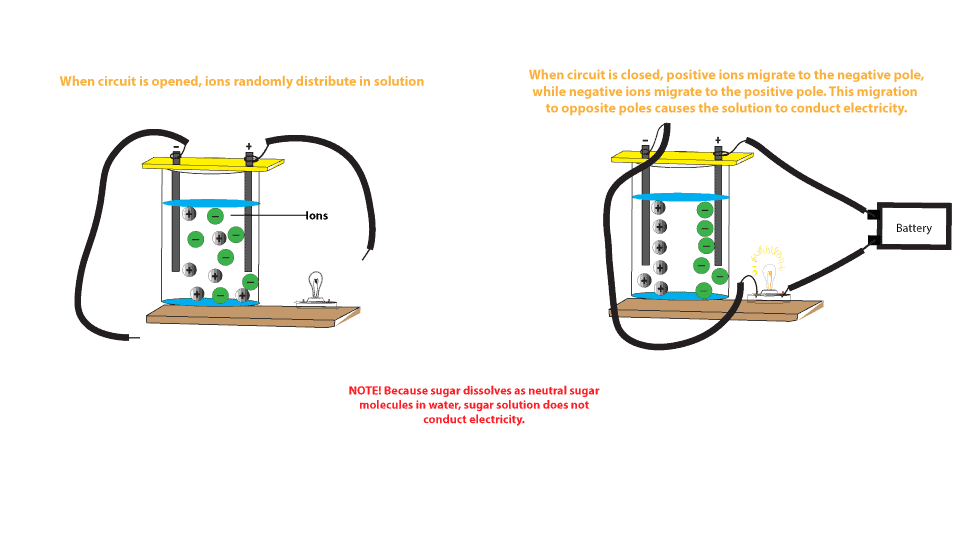
Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T
Physical Properties Of Ionic Compounds Ck 12 Foundation

Eight Arch Flash Safety Tips Arc Flash Flash Arc

This Yarn Conducts Electricity Wearable Electronics Smart Textiles Wearable Tech

Which Substance When Dissolved In Water Will Conduct An Electrical Current Science Project Education Com
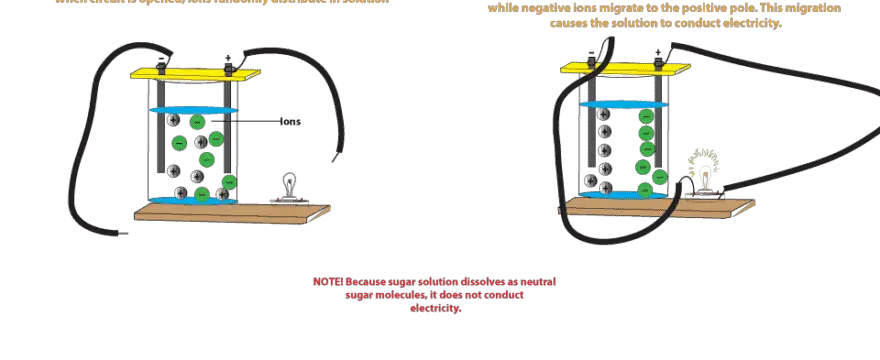
Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T

Another Aluminum Copper Interconnection That Almost Resulted In A Fire Fortcollinselectrician Fort Collins Homeowner Fire Hazard
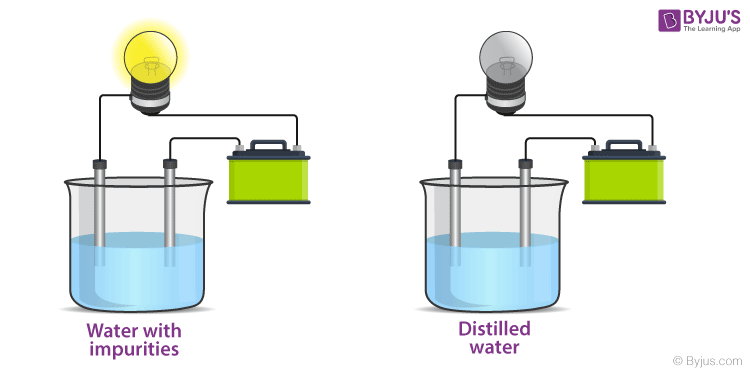
Electricity And Conduction Of Electricity Ionic And Covalent

Swot Analysis A Simple Way To Find Your Competitive Edge Plus A Free Template Swot Analysis Analysis Economy

Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T

Schematic Diagram Of Dye Sensitized Solar Cell Dssc Assembly In 2021 Solar Photovoltaic System Solar Power

Post a Comment for "Would The Resulting Solution Conduct Electricity"